Clinical Trials
Neurology & Psychiatry
She thought neurological symptoms would take away her work.
She just designed the fall line.
That is what drives us. Experience, Rho.

When it comes to the neurologic and psychiatric therapeutic areas, history would have us believe the future of these clinical trials is going to be mired in more of the same – failure.
Over the past decade, 99% of Alzheimer’s drug trials have failed and the likelihood of approval in neuropsychiatry indications is barely 6%. And with nearly a quarter of all Americans having at least one CNS disorder and the majority of effective treatments dating back to the 1950s, the need for better CNS disease research solutions globally is clear.
The way we see it, past failure isn’t a problem. It’s an opportunity.
An opportunity to ask ‘why?’ When history tells us that participant compliance is one of the biggest challenges to the success of CNS clinical trials, we listen, learn, and adapt in a way that only a neuroscience CRO could.
An opportunity to improve participant retention and study adherence by creating a trial experience that’s adaptable and as smooth as possible. By incorporating patient and caregiver needs and concerns into the initial study design process, as well as decentralized clinical trial needs, we are able to mitigate and minimize their burden before it becomes a problem – ensuring participants are fully engaged at each and every step of our CNS clinical research.
An opportunity to unite our global team of experts. With over 130 years of collective experience in the central nervous system (CNS) therapy area, including more than 350 clinical studies, 3,000 sites and 70,000 patients, no matter what challenges may arise during the development of your drug … our collaborative team of experts stand ready to utilize their learnings to enhance the success of your drug development program.
An opportunity to utilize the past to advance our future. Our cross-functional collaborative approach as a CNS focused CRO allows us to identify challenges earlier, de-risk your development strategy, and eliminate the missteps and delays often caused by silos and handoffs – ultimately ensuring a consistent program that’s flexible and runs smarter and more efficiently.
An opportunity to set a higher standard for CNS clinical research by overcoming past challenges and accelerating the patient path to relief and your path to approval.
And the perfect opportunity to do what we do best – create a better experience for our partners within their central nervous system (CNS) therapy area.
Global Reach
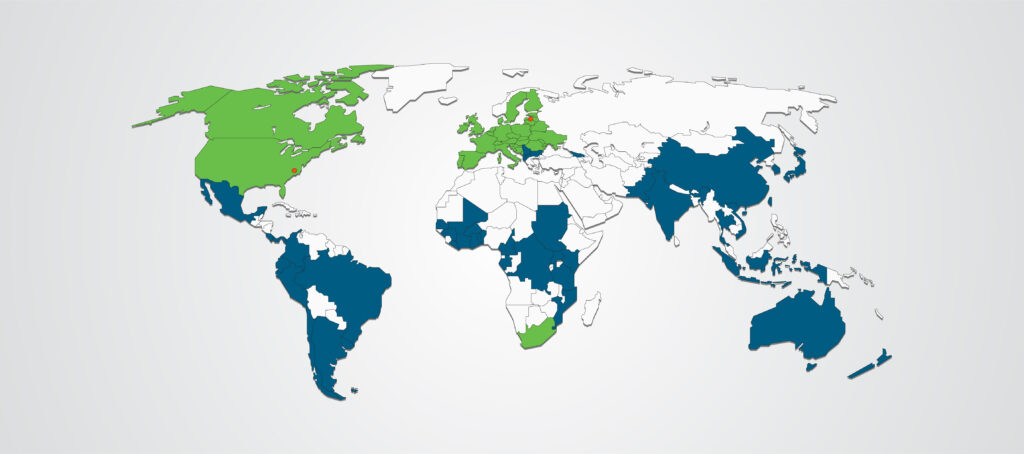
Our global client solutions will ensure you reach your patients, wherever they are. With international capabilities that span 5 continents, we provide full-service capabilities to execute your clinical development programs and trials across the globe.
I have been conducting mental health trials for over 20 years and my experience with Rho is phenomenal. Rho stands out far above other CROs as their teams are truly dedicated and committed to sites, understanding protocol complexities within mental health, and patient safety. With Rho, I have worked with the same CRA throughout a trial when industry norm is having 3-5 CRAs over the course of a trial. Rho CRAs have been very experienced and always responsive to study needs. I highly recommend Rho to any Sponsor.
Samantha Bostrom, MD/PI/Owner, The Focus Center for Clinical Research
Together, let’s build upon the shoulders of those brave enough to dare before us and deliver the long-awaited hope to the millions that are counting on us. We proudly invite you to experience Rho.

Why Choose Rho?
- More than a dozen articles in the area of CNS clinical research in prestigious peer-reviewed journals, including The Journal of the American Medical Association (JAMA), The Lancet Neurology, The Journal of Pediatric Psychology, and The Journal of the International Neuropsychological Society.
- An outstanding track record starting up and executing CNS clinical trials
- Excellent relationships with successful sites that will help you meet your enrollment goals
- Expertise managing and distributing Schedule II drugs
- Experience at FDA meetings handling missing data problems—a common issue in CNS trials because of study dropouts and missed assessment
Several successful 505(b)(1) submissions
3 recent 505(b)(2) submissions
Our Expertise
350+
clinical trials
5,000+
clinical sites
16
countries
Indications Expertise
-
Commonly used scales expertise
- Anxiety Rating Scales (e.g. HAM-A, STAI, Y-BOCS)
- Bipolar & Schizophrenia Scales (BPRS, YMRS, PANSS, SAPS/SANS)
- Cognitive Rating Scales (ADAS-cog, BLS-D, MMSE)
- Depression Rating Scales (HAM-D, MADRS, BDI)
- Diagnostic Scales (e.g., DIS, SCID, MINI)
- Quality of Life Scales (Q-LES-Q)
- Rating Scales for Children (CBCL, SKAMP, CRS)
- Suicide Rating Scales (C-SSRS, STS)
-
Central Nervous Systems (CNS) indications expertise
- Acute Pain
- ADHD, including Analog Classroom Assessments
- Alzheimer’s Disease
- Autism
- Bipolar Disorder
- Chronic Pain
- Depression/Major Depressive Disorder
- Memory Impairment
- Mild Cognitive Impairment
- Multiple Sclerosis
- Narcolepsy
- Opioid Addiction
- Parkinson’s Disease
- Schizophrenia
- Seizures, including Epilepsy
- Spasticity
- Stimulant Abuse
- Stroke
-
Psychiatric Disorder indications expertise
- Addiction
- Adjustment Disorder
- ADHD
- Alcohol/Substance Abuse
- Anxiety
- Bipolar Disorder
- Borderline Personality Disorder
- Childhood Disorders including Autism and Autism Spectrum Disorders
- Depression
- PTSD
- Schizophrenia
- Seasonal Affective Disorder
Featured Experts

Anna Pinsky, M.D., Ph.D.
Medical Director

Peter Schmidt, Ph.D.
Chief Scientific Officer

Nancy Yovetich, Ph.D.
Principal Research Scientist

Natalia Marder, M.D., Ph.D.
Senior Director, Project Delivery

Inese Nagle, M.D.
Enrollment Liaison Manager

Matt Healy
Senior Vice President, Global Clinical Operations

Rob Woolson
Senior Vice President, Regulatory Strategy, Biometrics & Technology

Ben Vaughn
Chief Strategist, Biostatistics & Protocol Design

Brett Gordon
Associate Vice President, Project Delivery

Amy Goodykoontz
Project Director

Candice French
Director, Project Delivery

Liene Savicka
Project Manager

Katarzyna Mierczyk
Clinical Trial Lead
Innovative Solutions to Overcome Enrollment Challenges in a CNS Trial
In this case study, our experts outline the implementation needed customize your enrollment solutions to catapult your development program.
Featured Content
Success Stories
CNS Trial Services
-
Clinical Project Management
-
Clinical Operations Monitoring
-
Global Reach
-
Phase I Clinical Development Services
-
Risk-Based Quality Management
-
Medical Monitoring
-
Data Management
-
Biostatistics
-
Statistical Programming
-
Pharmacovigilance
-
Regulatory Operations
-
Medical Writing
-
Data Standards
-
Quality Assurance
